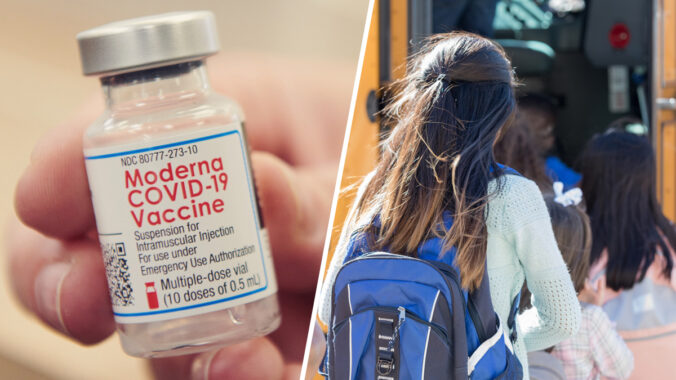
Moderna just launched its ‘KidCOVE’ study, the company’s first Covid-19 vaccine trial for kids.
The trial aims to test the efficacy of mRNA-1273, Moderna’s Covid-19 vaccine candidate, in kids between 6 months and 12 years. Moderna also hopes to eventually enroll 6,750 child participants from both the US and Canada.
Moderna CEO Stéphane Bancel commented:
We are pleased to begin this Phase 2/3 study of mRNA-1273 in healthy children in the U.S. and Canada and we thank NIAID and BARDA for their collaboration. It is humbling to know that 53 million doses have been administered to people in the U.S. We are encouraged by the primary analysis of the Phase 3 COVE study of mRNA-1273 in adults ages 18 and above and this pediatric study will help us assess the potential safety and immunogenicity of our COVID-19 vaccine candidate in this important younger age population.
Check out Moderna’s press release.